GCC Liquid Biopsy Market Size and Forecast 2025–2033
GCC’s Shift Toward Precision Oncology Fuels a High-Growth Era for Non-Invasive Cancer Diagnostics
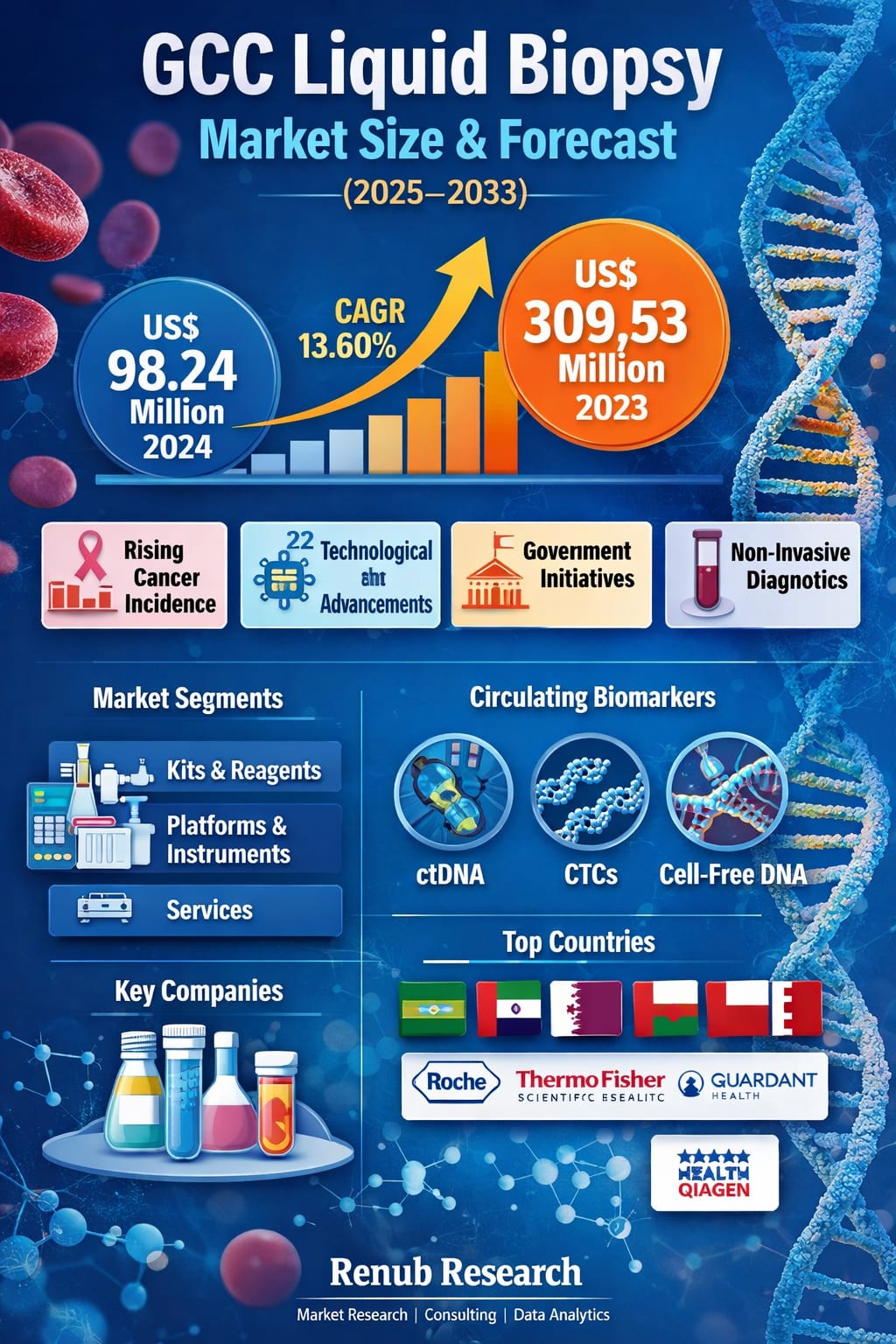
The GCC Liquid Biopsy Market is entering a decisive growth phase, driven by rising cancer incidence, increasing demand for non-invasive diagnostics, and rapid progress in genomic technologies. According to Renub Research, the market is expected to reach US$ 309.53 million by 2033, up from US$ 98.24 million in 2024, registering a strong CAGR of 13.60% from 2025 to 2033. This growth reflects a broader transformation in how cancer is diagnosed, monitored, and treated across the Gulf region.
Liquid biopsy, a non-invasive diagnostic technique, analyzes biomarkers found in bodily fluids such as blood, urine, or saliva. These biomarkers include circulating tumor DNA (ctDNA), RNA, circulating tumor cells (CTCs), and exosomes. Unlike traditional tissue biopsies, which are invasive, time-consuming, and sometimes risky for patients, liquid biopsy offers a faster, safer, and more patient-friendly alternative. It enables clinicians to detect genetic mutations, track disease progression, monitor treatment response, and identify minimal residual disease or recurrence—all through a simple blood draw.
In the GCC, where healthcare systems are rapidly modernizing and governments are investing heavily in advanced medical technologies, liquid biopsy is increasingly seen as a cornerstone of precision medicine. The region’s focus on early diagnosis, personalized treatment, and improved cancer outcomes is creating fertile ground for the expansion of this market.
Understanding the GCC Liquid Biopsy Market Landscape
At its core, the liquid biopsy market in the GCC is shaped by the convergence of three major forces: rising cancer burden, technological innovation, and policy-level support for advanced healthcare solutions. Cancer remains one of the leading causes of mortality in the Middle East, and its incidence is steadily increasing due to factors such as aging populations, lifestyle changes, and environmental influences.
Early detection plays a critical role in improving survival rates, and this is where liquid biopsy offers a compelling advantage. By enabling real-time, repeatable, and minimally invasive testing, liquid biopsy allows clinicians to stay ahead of disease progression and adapt treatment strategies more effectively. This capability is especially valuable in oncology, where tumors evolve over time and resistance to therapies can develop.
The GCC market is also benefiting from the growing adoption of next-generation sequencing (NGS), digital PCR, and advanced bioinformatics tools, which significantly enhance the sensitivity and accuracy of liquid biopsy tests. As these technologies become more accessible, their clinical utility continues to expand beyond oncology into areas such as reproductive health and other therapeutic applications.
Key Growth Drivers Shaping the Market
Rising Cancer Incidence
One of the most powerful drivers of the GCC liquid biopsy market is the rising incidence of cancer across the region. The burden of cancer is increasing at an alarming rate, and early diagnosis has become a public health priority. In several Middle Eastern countries, gaps in oncology infrastructure and late-stage diagnosis remain major challenges, making non-invasive and easily repeatable diagnostic tools even more valuable.
Liquid biopsy offers a practical solution by enabling earlier detection and continuous monitoring without the need for repeated invasive procedures. This not only improves patient comfort but also enhances clinical decision-making. As awareness of these benefits grows among healthcare providers and patients alike, adoption rates are expected to rise steadily across the GCC.
Advancements in Genomic and Diagnostic Technologies
Technological progress is another critical growth engine for the market. Innovations in NGS, digital PCR, and microfluidics have dramatically improved the ability to detect extremely low levels of circulating tumor DNA and other biomarkers in blood samples. These advancements make it possible to identify cancer-related mutations with greater precision, even at very early stages of the disease.
Moreover, the integration of artificial intelligence and advanced data analytics into diagnostic workflows is further enhancing the clinical value of liquid biopsy. These tools help interpret complex genomic data, support personalized treatment decisions, and improve overall diagnostic accuracy. As technology continues to evolve, the clinical applications of liquid biopsy in the GCC are likely to broaden significantly.
Government Support and Healthcare Investments
Government initiatives and public-sector investments are playing a vital role in accelerating market growth. Across the GCC, healthcare modernization is a strategic priority, with strong emphasis on innovation, research, and advanced diagnostics—particularly in oncology.
For example, national healthcare strategies in countries like Saudi Arabia and the UAE are focused on expanding specialized cancer care centers, strengthening research ecosystems, and promoting the adoption of cutting-edge medical technologies. Partnerships between healthcare authorities, private providers, and international technology companies are also helping to bring liquid biopsy solutions into mainstream clinical practice.
These policy-level efforts are not only improving access to advanced diagnostics but also creating a supportive environment for market expansion, research, and commercialization of new liquid biopsy technologies.
Challenges Facing the GCC Liquid Biopsy Market
High Cost of Technology
Despite its strong growth prospects, the market faces a significant challenge in the form of high technology costs. Advanced diagnostic platforms such as NGS and digital PCR require substantial investments in equipment, reagents, and skilled personnel. These costs can limit adoption, particularly in smaller hospitals, clinics, and public healthcare settings.
In addition, limited or inconsistent insurance coverage for advanced molecular diagnostics can further restrict patient access. Until costs come down and reimbursement frameworks become more favorable, price sensitivity is likely to remain a barrier to faster market penetration.
Regulatory Hurdles
Regulatory complexity is another obstacle. The GCC region lacks a fully harmonized regulatory framework for advanced diagnostic technologies, leading to variations in approval processes and compliance requirements across different countries. This fragmented landscape can delay product launches and increase the cost and complexity of regional expansion for manufacturers.
Clearer regulatory guidance and more streamlined approval pathways would help accelerate innovation and adoption, making it easier for new liquid biopsy solutions to reach healthcare providers and patients across the region.
Country-Level Market Insights
Saudi Arabia Liquid Biopsy Market
Saudi Arabia represents one of the most promising markets in the GCC for liquid biopsy. The country’s Vision 2030 initiative has placed healthcare transformation at the center of national development, leading to increased investment in hospitals, research centers, and advanced diagnostic infrastructure.
The adoption of NGS and digital PCR technologies is improving the accuracy and sensitivity of cancer diagnostics, and specialized oncology centers are increasingly incorporating liquid biopsy into clinical workflows. While challenges such as high costs and variable awareness remain, the long-term outlook is positive, with liquid biopsy expected to play a growing role in early cancer detection and disease monitoring.
UAE Liquid Biopsy Market
The UAE is emerging as a regional hub for advanced medical technologies, and liquid biopsy is no exception. Rising healthcare spending, expanding private and public healthcare infrastructure, and a strong focus on personalized medicine are driving market growth.
The increasing incidence of cancer and the demand for non-invasive diagnostic solutions are encouraging hospitals and diagnostic laboratories to adopt liquid biopsy techniques for both early detection and therapy monitoring. As the UAE continues to position itself as a leader in healthcare innovation, the role of liquid biopsy in precision oncology is set to expand further.
Oman Liquid Biopsy Market
Oman’s liquid biopsy market is growing steadily, supported by increased awareness of early cancer detection and ongoing investments in healthcare infrastructure. As cancer cases rise, the need for accurate, less invasive diagnostic tools is becoming more evident.
Liquid biopsy offers clear advantages over traditional tissue biopsies in terms of patient comfort, safety, and monitoring efficiency. As Oman continues to strengthen its healthcare system and research capabilities, liquid biopsy is expected to become an increasingly important component of cancer care.
Market Segmentation Overview
By Product
Kits & Reagents
Platforms & Instruments
Services
By Application
Cancer Therapeutic Application
Reproductive Health
Other Therapeutic
By Circulating Biomarkers
Circulating Tumor Cells (CTCs)
Circulating Tumor DNA (ctDNA)
Cell-free DNA (cfDNA)
Extracellular Vesicles
Other Biomarkers
By End User
Hospitals
Diagnostic Laboratories
Point-of-care Testing
Academic Institutes
Others
By Country
Saudi Arabia
UAE
Kuwait
Qatar
Oman
Bahrain
Competitive Landscape and Company Analysis
The GCC liquid biopsy market features a mix of global diagnostics leaders and specialized biotechnology firms, all competing to expand their footprint in this high-growth region. According to Renub Research, key companies covered in the market analysis include:
F. Hoffmann-La Roche Ltd.
Bio-Rad Laboratories
Thermo Fisher Scientific Inc.
Johnson & Johnson
Guardant Health Inc.
QIAGEN N.V.
Sysmex
These companies are analyzed across four dimensions: Overview, Key Persons, Recent Developments, and Financial Insights. Their strategies typically focus on product innovation, strategic partnerships, geographic expansion, and integration of advanced genomic technologies to strengthen their market positions.
As competition intensifies, innovation in test accuracy, turnaround time, and cost efficiency is expected to become a key differentiator in the GCC market.
Outlook: A Transformational Decade Ahead
Looking ahead, the GCC liquid biopsy market is poised for sustained and robust growth through 2033. The combination of rising cancer incidence, expanding healthcare investments, technological breakthroughs, and a strong push toward precision medicine creates a powerful foundation for long-term market expansion.
While challenges related to cost and regulation remain, ongoing innovation and policy support are likely to gradually ease these barriers. As awareness grows among clinicians and patients, liquid biopsy is expected to move from a specialized diagnostic tool to a mainstream component of cancer care across the GCC.
Final Thoughts
The projected rise of the GCC Liquid Biopsy Market from US$ 98.24 million in 2024 to US$ 309.53 million by 2033, at a CAGR of 13.60%, underscores a fundamental shift in the region’s approach to cancer diagnosis and management. Liquid biopsy is no longer just an emerging technology—it is becoming a strategic pillar of modern, patient-centric, and precision-driven healthcare.
As GCC countries continue to invest in advanced medical infrastructure and embrace genomic medicine, liquid biopsy will play a critical role in improving early detection, optimizing treatment strategies, and ultimately enhancing patient outcomes. For healthcare providers, policymakers, and industry players alike, the coming decade represents a pivotal opportunity to shape the future of non-invasive cancer diagnostics in the Gulf region.






Comments
There are no comments for this story
Be the first to respond and start the conversation.