Medical Writing Market Growth, Segments & Forecast 2025–2033 | Renub Research
Rising clinical trials, stricter regulations, and global drug development push medical writing into a high-growth decade
Medical Writing Market
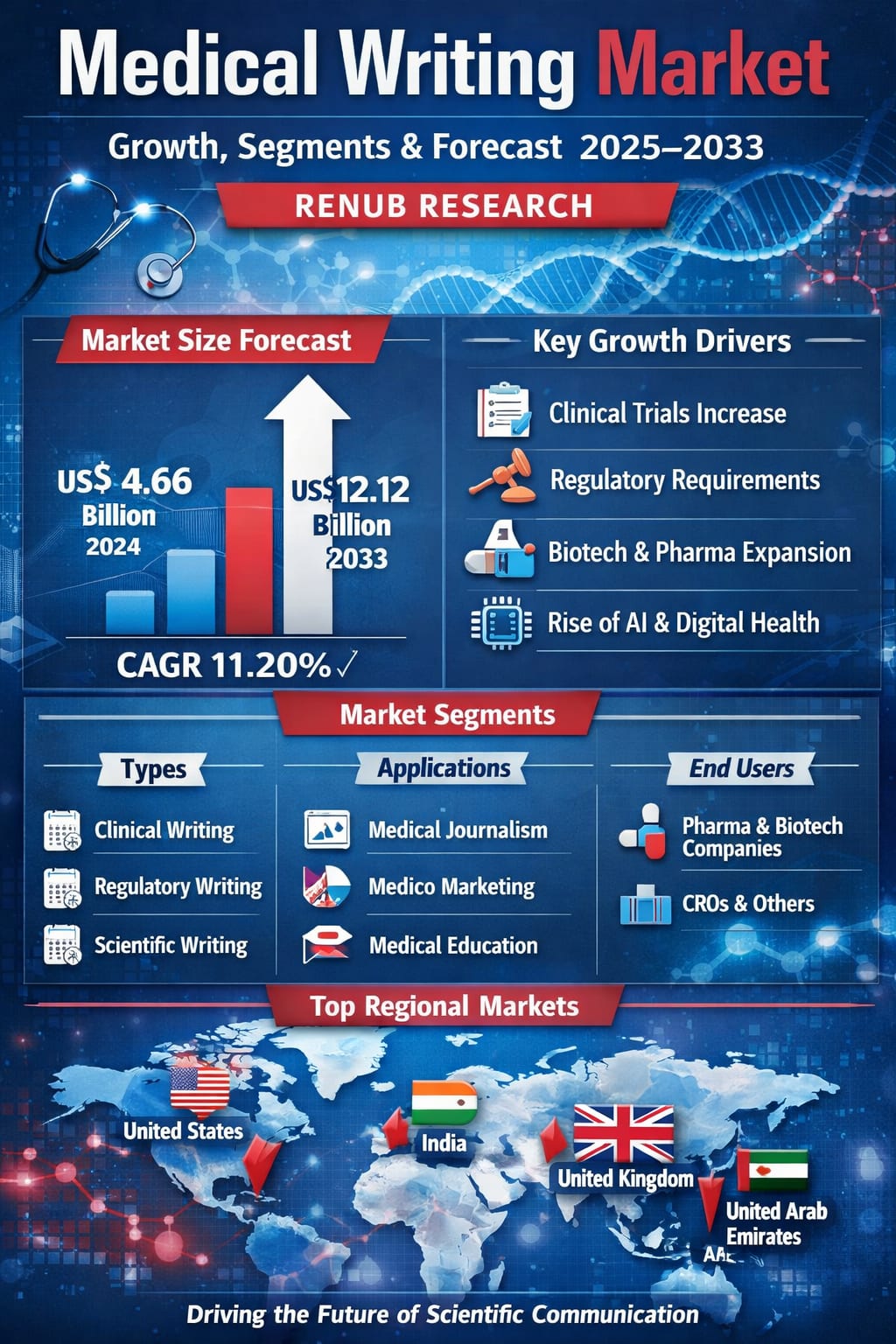
The global Medical Writing Market is entering a powerful growth phase, driven by the rapid expansion of the pharmaceutical and biotechnology industries, the surge in clinical trials, and the increasing complexity of regulatory requirements worldwide. According to Renub Research, the medical writing market was valued at US$ 4.66 billion in 2024 and is projected to reach US$ 12.12 billion by 2033, growing at a robust CAGR of 11.20% from 2025 to 2033.
Medical writing plays a crucial role in the modern healthcare ecosystem. It bridges the gap between complex scientific data and the stakeholders who rely on that information—regulatory authorities, healthcare professionals, researchers, and even patients. From clinical study reports and regulatory submissions to journal articles, educational materials, and marketing content, medical writers ensure that scientific information is accurate, clear, compliant, and usable.
As drug development becomes more global, more data-driven, and more tightly regulated, the demand for specialized medical writing services is rising sharply. The industry is also benefiting from trends such as outsourcing, digital publishing, personalized medicine, and the growing role of artificial intelligence in content development and documentation workflows.
Global Medical Writing Industry Overview
Medical writing refers to the professional creation of scientific documents related to medicine, healthcare, and drug development. These documents include clinical trial protocols, clinical study reports, regulatory submissions, scientific manuscripts, continuing medical education (CME) materials, and healthcare marketing content. The primary objective of medical writing is to communicate complex scientific and clinical information in a way that is precise, compliant, and understandable to different audiences.
Medical writers work closely with researchers, clinicians, regulatory experts, statisticians, and marketing teams. Their role is not just linguistic but also technical—requiring a strong understanding of medical science, clinical research processes, and global regulatory guidelines. Accuracy, consistency, and compliance are critical, as even small errors can lead to regulatory delays or misinterpretation of scientific results.
The importance of medical writing has increased significantly over the past decade. The volume of clinical research has grown, regulatory scrutiny has intensified, and scientific publishing has expanded rapidly in digital formats. At the same time, healthcare companies are under pressure to bring products to market faster while maintaining strict documentation standards. All these factors have positioned medical writing as a strategic function rather than just a support service.
Market Size and Growth Outlook
Renub Research estimates that the Medical Writing Market will grow from US$ 4.66 billion in 2024 to US$ 12.12 billion by 2033, reflecting a CAGR of 11.20% during the forecast period from 2025 to 2033.
This strong growth trajectory is being fueled by several long-term structural trends:
Expansion of the pharmaceutical, biotechnology, and medical device industries
Rising number of clinical trials and drug approvals
Increasing regulatory documentation requirements
Growing preference for outsourcing medical writing services
Rapid growth in scientific publishing and digital health communication
Technological advancements, including AI-assisted writing and data management tools
Increasing focus on personalized medicine and real-world evidence
Together, these factors are reshaping how medical content is created, managed, and delivered across the global healthcare industry.
Growth Drivers for the Medical Writing Market
Rising Number of Clinical Trials and Drug Approvals
One of the most important growth drivers for the medical writing market is the steady increase in clinical trials and new drug approvals worldwide. Each clinical trial generates a massive volume of documentation, including study protocols, investigator brochures, clinical study reports, patient narratives, and regulatory submission dossiers.
As pharmaceutical and biotech companies expand their research pipelines, the demand for high-quality, compliant documentation continues to rise. Regulatory authorities across the world require detailed, standardized, and transparent reporting of clinical data, making professional medical writing services indispensable.
In addition, the growth of complex trial designs—such as adaptive trials, real-world evidence studies, and multi-country trials—has further increased the need for experienced medical writers who can manage sophisticated documentation requirements.
Rising R&D Activities
Increased research and development (R&D) spending by pharmaceutical, biotechnology, and medical device companies is another major catalyst for market growth. As companies invest more in innovation, the volume of scientific data being generated is rising rapidly.
This data must be translated into regulatory submissions, scientific publications, and internal research documents. Medical writers play a critical role in ensuring that these documents are accurate, consistent, and aligned with regulatory standards.
The industry has also seen strategic acquisitions and expansions aimed at strengthening scientific communication capabilities. For example, in January 2023, Cactus Communications acquired FlexSteel Technologies Holdings, Inc. in a deal valued at US$ 621 million upfront, with an additional potential earn-out. While FlexSteel focuses on spoolable pipe technologies, the move highlights the broader trend of companies expanding their expertise and service portfolios in technical and scientific communication.
Globalization of Drug Development
Drug development is no longer confined to a single country or region. Clinical trials are increasingly conducted across multiple geographies, and regulatory submissions often need to be prepared for several authorities simultaneously, such as the FDA, EMA, and other national agencies.
This globalization has significantly increased the demand for medical writing services that can ensure consistency, accuracy, and compliance across different regulatory frameworks. In many cases, documents must also be adapted for local languages and cultural contexts, further increasing the complexity of medical writing projects.
Reflecting this trend, BioMed Solutions launched an innovative medical writing training program in April 2023 to equip professionals with the skills needed to succeed in this rapidly evolving global market. Such initiatives underline the growing strategic importance of medical writing in international drug development.
Challenges in the Medical Writing Market
Maintaining Consistency and Quality
One of the key challenges in medical writing is maintaining consistency and quality across large, complex projects. Clinical and regulatory documents often involve multiple contributors, including clinicians, statisticians, and regulatory experts. This increases the risk of inconsistencies in terminology, data presentation, and tone.
Ensuring high-quality output requires rigorous quality control processes, including multiple rounds of review, strict version control, and adherence to templates and style guides. While these processes are essential, they are also time-consuming and resource-intensive, adding pressure to project timelines and budgets.
Shortage of Skilled Medical Writers
Despite strong demand, the industry faces a shortage of skilled medical writers who possess both scientific expertise and regulatory writing experience. Medical writing requires a unique combination of skills, including knowledge of clinical research, understanding of regulatory guidelines, and strong communication abilities.
As the volume of clinical trials and regulatory submissions continues to grow, competition for qualified professionals is intensifying. This talent gap can lead to project delays, higher costs, and increased workload for existing teams.
Regional Market Insights
United States Medical Writing Market
The United States represents one of the largest and most mature markets for medical writing, driven by its strong pharmaceutical, biotechnology, and healthcare research ecosystem. The country conducts a significant share of global clinical trials, particularly in therapeutic areas such as oncology, cardiology, and neurology.
According to data from clinicaltrials.gov, as of July 2022, there were approximately 10,548 Phase III cancer studies, 6,349 cardiology studies, and 947 neurology studies ongoing or registered. Each of these studies generates extensive documentation requirements, fueling demand for professional medical writing services.
Over the forecast period, the continued growth in clinical research activity, along with stringent regulatory standards, is expected to keep the U.S. market at the forefront of the global medical writing industry.
India Medical Writing Market
India has emerged as a major hub for medical writing services, supported by its large pool of highly skilled, English-speaking professionals and its strong presence in clinical research and pharmaceutical manufacturing. The country is widely recognized as a preferred destination for outsourcing medical writing tasks, including clinical study reports, regulatory documentation, and scientific publications.
Cost advantages, improving infrastructure, and the adoption of advanced technologies such as artificial intelligence are further strengthening India’s competitive position. As more global pharmaceutical and biotech companies look to optimize costs and scale operations, India’s medical writing market is expected to continue its rapid expansion.
United Kingdom Medical Writing Market
The United Kingdom benefits from a well-established healthcare system, a strong pharmaceutical and biotechnology sector, and a highly skilled workforce. The increasing number of clinical trials and the growing need for precise, compliant documentation are key drivers of market growth in the country.
Clinical writing remains the largest revenue-generating segment, while regulatory writing is also witnessing steady growth due to evolving compliance requirements. The UK’s strong research ecosystem and international regulatory influence make it an important player in the global medical writing landscape.
United Arab Emirates Medical Writing Market
The medical writing market in the United Arab Emirates (UAE) is expanding steadily, supported by investments in healthcare infrastructure, rising clinical research activity, and the country’s growing role as a regional healthcare and medical tourism hub.
The adoption of digital health technologies and the focus on meeting international regulatory standards are also increasing the demand for specialized medical writing services. As the UAE continues to develop its healthcare and research capabilities, the need for high-quality scientific and regulatory documentation is expected to rise further.
Medical Writing Market Segmentation
By Type
Clinical Writing
Regulatory Writing
Scientific Writing
Others
By Application
Medical Journalism
Medical Education
Medico Marketing
Others
By End Use
Contract Research Organizations & Others
Medical Device / Pharmaceutical & Biotechnology Companies
Country-Level Market Coverage (25 Viewpoints)
North America
United States
Canada
Europe
France
Germany
Italy
Spain
United Kingdom
Belgium
Netherlands
Turkey
Asia Pacific
China
Japan
India
Australia
South Korea
Thailand
Malaysia
Indonesia
New Zealand
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
United Arab Emirates
Competitive Landscape and Key Players
All major companies have been analyzed from four perspectives:
Company Overview
Key Persons
Recent Developments & Strategies
Sales Analysis
Key Players Include:
Parexel International Corporation
Trilogy Writing & Consulting GmbH
Freyr
Cactus Communications
Labcorp Drug Development
IQVIA Holdings Inc.
Omics International
Synchrogenix
These companies are focusing on expanding service portfolios, investing in technology, strengthening global delivery models, and forming strategic partnerships to gain a competitive edge in the rapidly evolving medical writing market.
Final Thoughts
The global Medical Writing Market is on a strong growth path, underpinned by the expansion of clinical research, increasing regulatory complexity, and the globalization of drug development. With the market expected to grow from US$ 4.66 billion in 2024 to US$ 12.12 billion by 2033 at a CAGR of 11.20%, medical writing is becoming a strategic pillar of the life sciences industry.
As healthcare innovation accelerates and documentation requirements continue to intensify, the role of medical writers will only become more critical. Companies that invest in skilled talent, advanced technologies, and global delivery capabilities will be best positioned to capitalize on the opportunities ahead. In this evolving landscape, medical writing is no longer just about documentation—it is about enabling faster, safer, and more transparent healthcare innovation worldwide.





Comments
There are no comments for this story
Be the first to respond and start the conversation.