North America Clinical Trials Market Size and Forecast 2025–2033
Rising R&D investments, chronic disease burden, and digital innovation are reshaping the future of clinical research across the region
Introduction
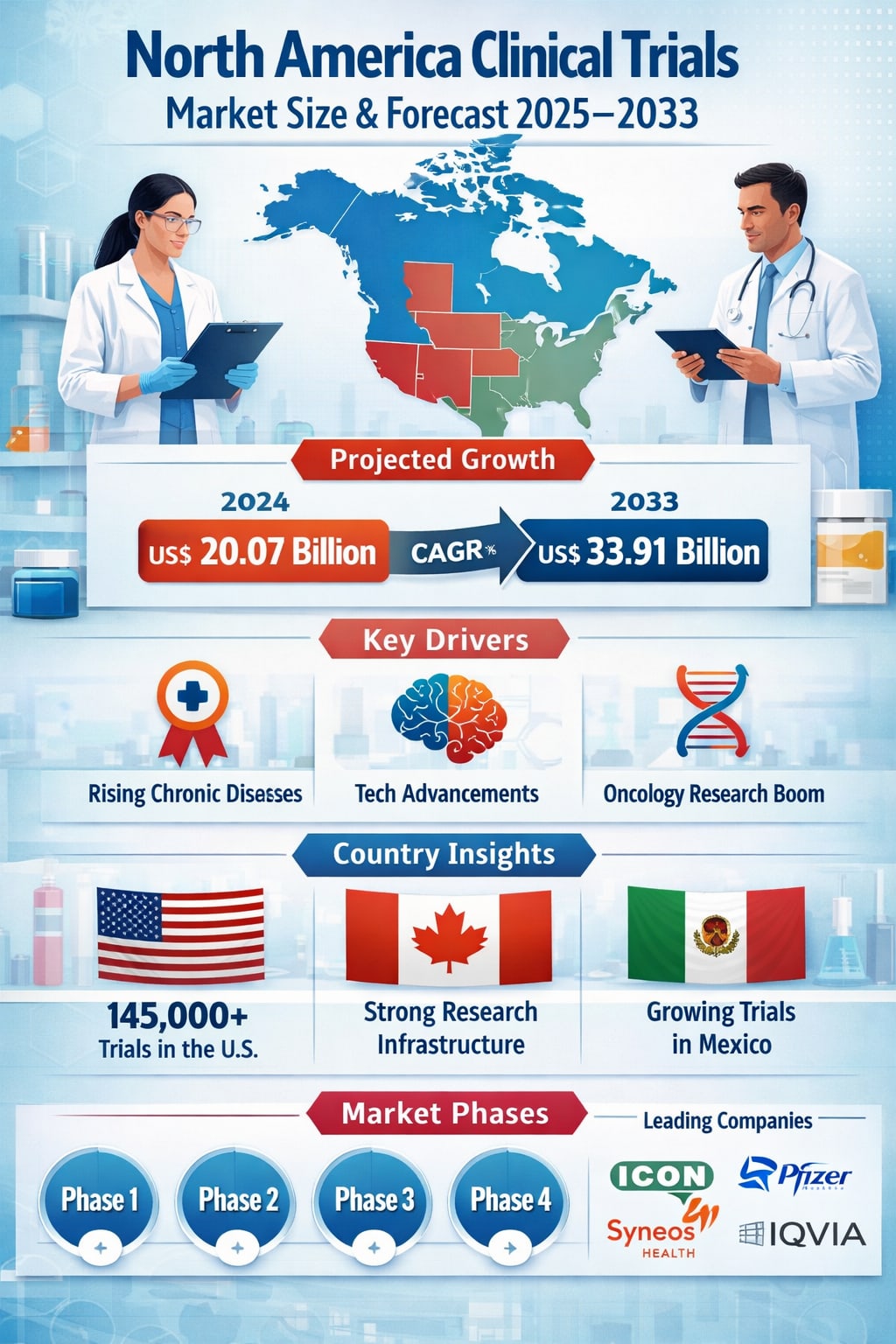
The North America Clinical Trials Market is entering a decisive growth phase as pharmaceutical and biotechnology companies race to develop safer, more effective, and more personalized treatments. According to Renub Research, the market is expected to grow from US$ 20.07 billion in 2024 to US$ 33.91 billion by 2033, expanding at a CAGR of 6.00% from 2025 to 2033. This steady rise reflects longer clinical trial cycles, increasing demand for innovative therapies, and the growing burden of chronic and infectious diseases such as cancer, diabetes, and post-pandemic viral conditions.
Clinical trials are the backbone of modern medicine. Every new drug, vaccine, or medical device must pass through multiple phases of testing before it can reach patients. In North America, a combination of strong research infrastructure, favorable funding environments, and advanced digital technologies has made the region a global hub for clinical research. At the same time, the market faces persistent challenges, including high operational costs, complex regulatory frameworks, and difficulties in patient recruitment.
Despite these hurdles, the outlook remains highly optimistic. The growing number of trials, rising R&D spending by pharmaceutical companies, and the increasing use of artificial intelligence, big data, and decentralized trial models are transforming how studies are designed and conducted. Together, these forces are setting the stage for a more efficient, patient-centric, and data-driven clinical research ecosystem across the United States, Canada, Mexico, and the rest of North America.
North America Clinical Trials Market Overview
The North American clinical trials industry plays a critical role in the global healthcare innovation pipeline. The region benefits from world-class hospitals, research universities, biotechnology clusters, and contract research organizations (CROs) that support every stage of drug development—from early discovery to post-marketing studies.
The United States dominates the regional landscape thanks to its strong healthcare infrastructure, deep capital markets, and robust public and private investment in medical research. Canada and Mexico are also emerging as attractive destinations for clinical trials due to their skilled workforce, supportive regulatory reforms, and cost advantages in certain areas.
Technological innovation is reshaping the sector at a rapid pace. Tools such as artificial intelligence, machine learning, wearable devices, and real-world data platforms are helping researchers design smarter trials, recruit patients more efficiently, and monitor outcomes in real time. The rise of decentralized and hybrid clinical trial models, accelerated during the COVID-19 era, has further expanded patient access and improved operational flexibility.
However, the industry is not without its challenges. Regulatory compliance remains complex, trial timelines are often lengthy, and the cost of conducting large, multi-center studies continues to rise. Even so, the strong demand for new therapies—especially in oncology, neurology, cardiology, and metabolic diseases—continues to fuel long-term growth in the North American clinical trials market.
Key Growth Drivers
Rising Prevalence of Chronic Diseases
One of the most powerful forces driving the clinical trials market in North America is the increasing burden of chronic diseases such as cancer, diabetes, cardiovascular disorders, autoimmune conditions, and neurological illnesses. An aging population, lifestyle changes, and environmental factors have all contributed to higher disease prevalence across the region.
Chronic diseases often require long-term management and innovative treatment approaches, which in turn fuels demand for continuous clinical research. Pharmaceutical companies and research institutions are under constant pressure to develop new drugs, biologics, and combination therapies that can improve patient outcomes and quality of life. Clinical trials are essential for validating the safety and effectiveness of these treatments, making them a central pillar of modern healthcare innovation.
As healthcare systems shift toward more personalized and precision-based medicine, the number of targeted therapies entering clinical development continues to rise. This trend further expands the volume and complexity of clinical trials, strengthening the market’s growth trajectory.
Advancements in Technology
Technology is fundamentally transforming how clinical trials are designed and executed in North America. Artificial intelligence (AI), machine learning (ML), and big data analytics are now widely used to analyze massive datasets, identify suitable trial participants, predict outcomes, and optimize study protocols.
Digital tools enable faster and more accurate decision-making, helping sponsors reduce trial durations, lower costs, and improve success rates. Remote monitoring devices, electronic health records, and mobile health applications allow researchers to collect real-time data from patients, even outside traditional clinical settings. This shift supports the growing adoption of decentralized and hybrid trial models, which improve patient convenience and expand access to diverse populations.
By improving efficiency, reducing errors, and enhancing data quality, technological innovation has become one of the most important growth engines for the North America clinical trials market.
Increased Investment in Oncology Research
Oncology remains one of the most active and competitive areas of clinical research in North America. The rising incidence of cancer and the urgent need for more effective treatments have led to a surge in funding from pharmaceutical companies, biotechnology firms, governments, and non-profit organizations.
Significant resources are being directed toward the development of immunotherapies, targeted therapies, cell and gene therapies, and personalized cancer treatments. Each of these approaches requires extensive clinical testing across multiple phases, driving up the number of ongoing trials in the region.
The strong focus on oncology not only increases the overall volume of clinical trials but also raises their scientific complexity, encouraging greater collaboration between sponsors, academic institutions, and CROs. As cancer continues to be a leading cause of mortality, oncology research is expected to remain a central pillar of the North American clinical trials market for years to come.
Challenges Facing the Market
High Operational Costs
One of the most significant challenges in the North America clinical trials market is the high cost of conducting studies. Expenses related to skilled personnel, advanced technology, regulatory compliance, data management, patient recruitment, and multi-site coordination can be substantial, especially for large Phase 3 and Phase 4 trials.
Smaller biotechnology companies and academic research institutions often struggle to bear these costs, forcing them to rely on partnerships, grants, or outsourcing to CROs. As trials become more complex and personalized, operational expenses continue to rise, placing pressure on sponsors to balance cost efficiency with data quality and regulatory requirements.
Regulatory Complexities
Regulatory compliance is another major hurdle. Agencies such as the U.S. Food and Drug Administration (FDA) enforce strict guidelines to ensure patient safety and data integrity. While these regulations are essential, they also increase administrative workloads, extend approval timelines, and raise overall trial costs.
Conducting multi-country or multi-site trials adds another layer of complexity, as sponsors must navigate different regulatory frameworks and ethical review processes. These challenges can delay trial initiation, disrupt timelines, and increase the risk of budget overruns, making regulatory management a critical focus area for the industry.
Country-Level Insights
United States Clinical Trials Market
The United States is the undisputed leader in the North American clinical trials market. It benefits from a vast network of research institutions, hospitals, CROs, and biotechnology hubs, along with strong government support from organizations such as the National Institutes of Health (NIH) and regulatory guidance from the FDA.
The country also hosts many of the world’s leading CROs, including Charles River Laboratories, ICON plc, Parexel, Syneos Health, and IQVIA, which play a key role in managing complex global trials. According to ClinicalTrials.gov, more than 145,000 studies were listed in the U.S. by December 2023, accounting for roughly 31% of all clinical trials worldwide.
High healthcare spending per capita, a large patient population, and strong innovation ecosystems continue to support market growth. However, the U.S. market also faces challenges such as high costs, regulatory complexity, and the ongoing need to improve patient diversity in clinical research.
Canada Clinical Trials Market
Canada has built a strong reputation as a reliable and efficient destination for clinical research. The country offers a skilled workforce, advanced healthcare facilities, and relatively streamlined ethics and regulatory approval processes, making it attractive to both domestic and international sponsors.
Clinical trials in Canada are particularly active in areas such as oncology, neurology, and infectious diseases. Government support and public-private partnerships play a key role in promoting research and innovation. Still, the market must address challenges related to patient recruitment, global competition, and the need for more inclusive trial populations.
Mexico Clinical Trials Market
Mexico is emerging as an increasingly important player in the North American clinical trials landscape. Lower operational costs, proximity to the United States, and regulatory improvements by COFEPRIS (Mexico’s health authority) have made the country an appealing destination for outsourced clinical research.
Faster approval timelines, growing adoption of digital health tools, and expanding healthcare infrastructure are helping Mexico attract more international trials. As decentralized and virtual trial models gain traction, Mexico’s role in regional and global clinical research is expected to continue growing.
Market Segmentation Overview
By Phases:
Phase 1
Phase 2
Phase 3
Phase 4
By Indications:
Autoimmune / Inflammation
Pain Management
Oncology
CNS Conditions
Diabetes
Obesity
Cardiovascular
Others
By Study Designs:
Interventional
Observational
Expanded Access
By Country:
United States
Canada
Mexico
Rest of North America
Competitive Landscape
The North America clinical trials market is highly competitive, with a mix of global pharmaceutical companies, CROs, and specialized service providers. Key players include:
ICON Plc
WuXi AppTec
SGS SA
Syneos Health
PRA Health Sciences Inc.
Pfizer Inc.
IQVIA
Medpace
These companies compete on the basis of service quality, technological capabilities, global reach, and expertise across different therapeutic areas. Strategic partnerships, acquisitions, and investments in digital platforms are common as firms seek to expand their capabilities and improve operational efficiency.
Future Outlook
Looking ahead, the North America clinical trials market is poised for steady and sustainable growth. The combination of rising disease burden, strong R&D pipelines, and rapid technological innovation will continue to drive demand for clinical research services across the region.
Decentralized trials, real-world evidence, and AI-driven analytics are expected to play an even greater role in shaping the future of the industry. At the same time, efforts to streamline regulations, reduce costs, and improve patient engagement will be critical for maintaining long-term momentum.
With Renub Research projecting the market to reach US$ 33.91 billion by 2033, the sector is clearly positioned as a cornerstone of the region’s healthcare and life sciences ecosystem.
Final Thoughts
The North America clinical trials market stands at the intersection of science, technology, and patient care. While challenges such as high costs and regulatory complexity remain, the region’s strong innovation culture, investment climate, and research infrastructure continue to support robust growth. As the industry evolves toward more digital, patient-centric, and data-driven models, clinical trials will remain a vital engine of medical progress—helping bring the next generation of life-saving therapies from the laboratory to the bedside.




Comments
There are no comments for this story
Be the first to respond and start the conversation.