United States Allergy Treatment Market Size & Forecast 2026–2034
How Rising Allergy Burden, Biologic Breakthroughs, and Preventive Healthcare Are Reshaping Treatment Demand
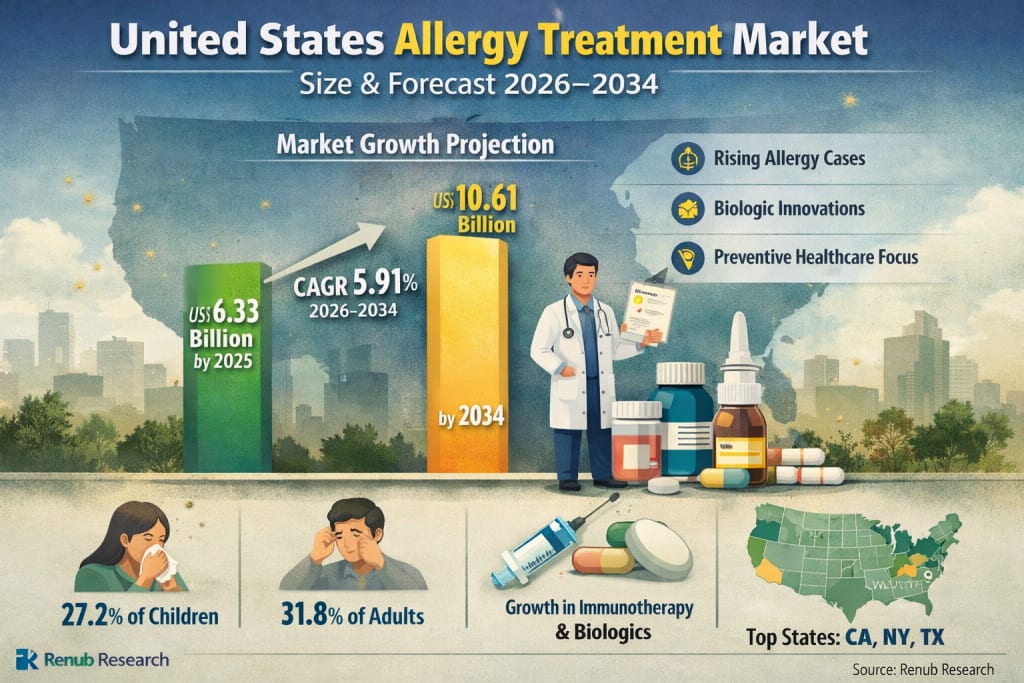
United States Allergy Treatment Market Outlook
The United States allergy treatment market is poised for steady and sustained expansion over the coming decade, reflecting the growing burden of allergic conditions and continuous therapeutic innovation. According to Renub Research estimates, the market is expected to grow from US$ 6.33 billion in 2025 to US$ 10.61 billion by 2034, registering a compound annual growth rate (CAGR) of 5.91% from 2026 to 2034.
Allergy treatment encompasses a broad range of medical interventions designed to prevent, manage, and relieve symptoms caused by hypersensitive immune responses. Common allergens include pollen, dust mites, animal dander, foods, insect venom, and certain medications. Treatment options span antihistamines, decongestants, corticosteroids, leukotriene modifiers, immunotherapy, and advanced biologic drugs that target specific immune pathways.
In the United States, allergies are among the most prevalent chronic conditions, affecting individuals across all age groups. Allergic rhinitis, asthma, food allergies, eczema, and eye allergies are increasingly diagnosed due to heightened awareness, environmental changes, and improved access to healthcare. With strong specialist availability, advanced diagnostics, and widespread patient education, the U.S. remains one of the most mature yet innovation-driven markets for allergy treatment worldwide.
Key Growth Drivers in the United States Allergy Treatment Market
Rising Prevalence of Allergic Conditions
The growing incidence of allergic diseases remains the single most important driver of market growth in the United States. Millions of Americans suffer from seasonal allergies, asthma, food allergies, atopic dermatitis, and allergic conjunctivitis. Environmental pollution, climate change, urban living, and prolonged exposure to allergens have contributed to increased diagnosis rates and disease severity.
Chronic and recurring symptoms significantly impact quality of life, productivity, and healthcare utilization, driving sustained demand for both prescription and over-the-counter allergy treatments. Early diagnosis and proactive disease management are increasingly emphasized to reduce emergency visits and hospitalizations.
Data from the CDC’s National Center for Health Statistics indicate that 27.2% of children and 31.8% of adults in the United States report allergy symptoms, representing well over 100 million individuals. This expanding patient pool continues to underpin long-term growth across medications, immunotherapy, and biologic therapies.
Innovation in Advanced and Biologic Therapies
Technological advancement has transformed allergy management in recent years. The introduction of biologic therapies targeting immunoglobulin E (IgE) and other immune pathways has reshaped treatment for patients with severe or treatment-resistant allergic conditions.
Compared with conventional therapies, biologics offer improved symptom control, reduced exacerbations, and better safety profiles. At the same time, immunotherapy has evolved beyond traditional injections, with sublingual tablets and oral formulations gaining acceptance among both clinicians and patients.
Pharmaceutical companies are investing heavily in research and development to introduce novel formulations, delivery methods, and personalized therapies. These innovations support premium pricing, higher adherence, and long-term treatment engagement.
A notable milestone occurred in February 2024, when the U.S. Food and Drug Administration approved Xolair (omalizumab) for immunoglobulin E-mediated food allergy in certain adults and children aged one year and older. The therapy reduces the risk of allergic reactions, including anaphylaxis, caused by accidental food exposure—marking a major breakthrough in food allergy management.
Increasing Awareness and Focus on Preventive Healthcare
Rising awareness of allergic diseases and their long-term consequences has encouraged patients to seek earlier and more consistent treatment. Public health campaigns, improved access to allergists, and expanded diagnostic testing have significantly increased identification rates.
Employers and insurance providers are also emphasizing preventive healthcare to reduce long-term costs associated with poorly controlled allergies. As a result, preventive approaches—such as routine medication use and long-term immunotherapy—are increasingly favored over episodic symptom relief.
In September 2025, Food Allergy Research & Education (FARE) launched a nationwide public service campaign aimed at empowering the 33 million Americans living with food allergies. Sponsored by Genentech and Novartis, the campaign highlighted education, community support, and proactive disease management, further reinforcing market demand.
Challenges Facing the U.S. Allergy Treatment Market
High Cost of Advanced Therapies
Despite innovation, affordability remains a significant challenge. Biologic therapies and long-term immunotherapy regimens are expensive and often require ongoing specialist care. Insurance coverage varies widely, and high out-of-pocket costs can deter patients from initiating or continuing treatment.
For healthcare providers and manufacturers, balancing innovation with accessibility is critical. Cost barriers may slow adoption rates for advanced therapies, particularly among underserved populations, potentially limiting overall market penetration.
Treatment Adherence and Long Therapy Duration
Many allergy treatments—especially immunotherapy—require multi-year commitment to achieve lasting benefits. Poor adherence due to inconvenience, side effects, or delayed symptom improvement remains a concern.
Injectable and oral regimens may feel burdensome, particularly for pediatric and elderly patients. Improving patient education, simplifying dosing schedules, and developing more convenient delivery systems are essential strategies for maximizing clinical outcomes and sustaining market growth.
Segment-Wise Market Insights
United States Eye Allergy Treatment Market
Eye allergy treatments are witnessing strong demand due to the high prevalence of seasonal and perennial allergic conjunctivitis. Common triggers include pollen, dust, pet dander, and pollution. Antihistamine eye drops, mast cell stabilizers, corticosteroids, and combination therapies dominate this segment.
Increased screen time and worsening air quality have intensified symptoms, driving higher utilization of both prescription and over-the-counter eye allergy medications across the country.
United States Food Allergy Treatment Market
Food allergy treatment in the U.S. is evolving beyond strict allergen avoidance. Growing awareness of the severity of food allergies—especially among children—has fueled demand for preventive and risk-reducing therapies.
Parents, caregivers, schools, and healthcare providers are increasingly proactive in managing food allergies through emergency preparedness, supportive therapies, and emerging biologic treatments that reduce reaction severity.
United States Anti-Allergy Drugs Market
Anti-allergy drugs remain a cornerstone of allergy management. Antihistamines, glucocorticoids, and leukotriene inhibitors are widely prescribed for both acute and chronic conditions. Over-the-counter availability has expanded access, supporting high sales volumes.
Combination therapies and extended-release formulations are gaining popularity due to improved convenience and efficacy, while strong brand recognition and continuous reformulation sustain competition.
United States Oral Allergy Treatment Market
Oral allergy medications—including tablets, syrups, and oral immunotherapy—are gaining traction due to ease of administration and improved patient compliance. These formulations are particularly attractive for children and patients seeking alternatives to injections.
Advances in formulation technology have improved drug stability and effectiveness, allowing oral treatments to expand across multiple allergy indications.
United States Allergy Treatment Retail Pharmacies Industry
Retail pharmacies play a critical role in allergy treatment distribution. Their extensive geographic presence, long operating hours, and availability of both prescription and OTC products make them the first point of care for many patients.
Pharmacists also influence treatment decisions through patient counseling and education. The growth of chain pharmacies and online prescription services has further strengthened this distribution channel.
Regional Market Highlights
California Allergy Treatment Market
California represents one of the largest state-level markets due to its dense population, diverse climate, wildfire exposure, and high pollution levels. Advanced healthcare infrastructure and proximity to pharmaceutical innovation hubs support rapid adoption of new therapies.
New York Allergy Treatment Market
Urban living, pollution, and seasonal allergens drive strong demand in New York. High patient awareness and access to specialists support sustained use of medications and immunotherapy through hospitals and retail pharmacies.
Washington Allergy Treatment Market
Seasonal pollen, mold, and moisture contribute to consistent demand in Washington. The state’s emphasis on preventive healthcare and strong medical infrastructure supports steady market growth.
New Jersey Allergy Treatment Market
High population density, proximity to major metropolitan areas, and strong insurance coverage make New Jersey a key market. The presence of leading pharmaceutical companies further enhances access to advanced treatments.
Market Segmentation Overview
By Type:
Eye Allergy, Skin Allergy, Food Allergy, Asthma, Rhinitis, Others
By Treatment:
Anti-Allergy Drugs, Immunotherapy
By Dosage Form:
Oral, Inhalers, Intranasal, Others
By Distribution Channel:
Hospital Pharmacies, Retail Pharmacies, Online Retailers, Others
By Geography:
California, Texas, New York, Florida, Illinois, Pennsylvania, Ohio, Georgia, New Jersey, Washington, North Carolina, Massachusetts, Virginia, Michigan, Maryland, Colorado, Tennessee, Indiana, Arizona, Minnesota, Wisconsin, Missouri, Connecticut, South Carolina, Oregon, Louisiana, Alabama, Kentucky, Rest of United States
Competitive Landscape
Key companies analyzed under five viewpoints—overview, key personnel, recent developments, SWOT analysis, and revenue analysis—include:
AbbVie Inc.
ALK-Abelló A/S
ALLERGOPHARMA GmbH & Co. KG
Almirall, S.A.
DBV Technologies
GSK plc
HAL Allergy B.V.
Novartis Pharmaceuticals Corporation
Teva Pharmaceutical Industries Ltd.
Viatris Inc.
Final Thoughts
The United States allergy treatment market is entering a phase of sustained, innovation-led growth. Rising allergy prevalence, expanding biologic therapies, and a stronger emphasis on preventive healthcare are reshaping how allergic conditions are managed nationwide. While cost and adherence challenges remain, continued investment in patient-centric solutions and advanced therapeutics is expected to drive long-term market expansion through 2034.






Comments
There are no comments for this story
Be the first to respond and start the conversation.